What to Know About Participating in a Clinical Trial
What to Know About Participating in a Clinical Trial
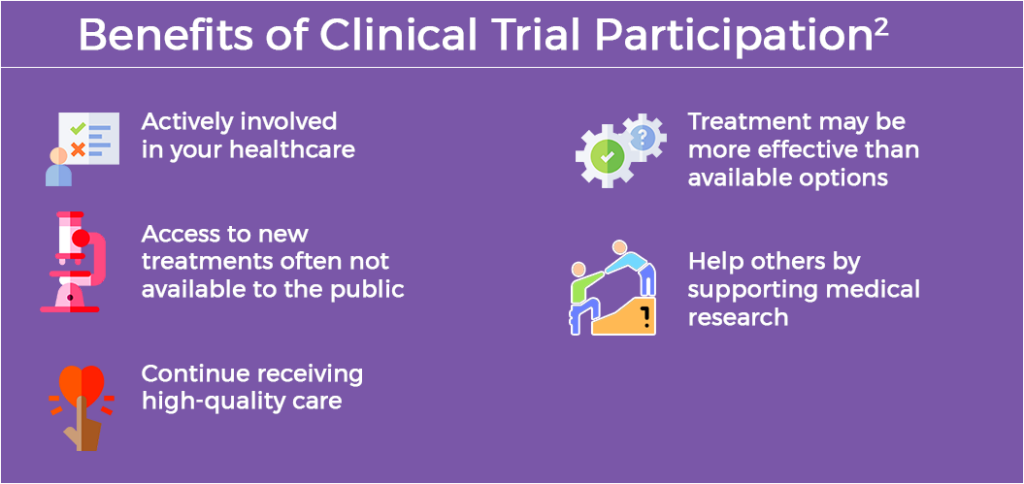
If eligible, it is important to consider participating in a clinical trial because research is key to making progress against cancer.1 Clinical trials have led to meaningful improvements in cancer treatments and have extended the lives of many patients with cancer.1 Of note, treatments are normally studied for safety and effectiveness for many years before being studied in humans in clinical trials.1 Taking part in a clinical trial adds to the knowledge about cancer and helps improve cancer care and outcomes for all.1
Discussing the details about clinical trial opportunities with your cancer care team can help you decide if participating in a trial is right for you. Recommended questions to ask an oncologist or other members of the cancer care team when deciding whether to participate in a clinical trial include3:
References
- National Cancer Institute. What are Clinical Trials? Reviewed November 3, 2024. https://www.cancer.gov/about-cancer/treatment/clinical-trials/what-are-trials
- Texas Oncology. Clinical Trials and Cancer Research. https://www.texasoncology.com/clinical-trials
- The U.S. Department of Health & Human Services. Questions to Ask About Volunteering for a Research Study. Last reviewed January 28, 2020. https://www.hhs.gov/ohrp/education-and-outreach/about-research-participation/questions-to-ask/index.html
ALL URLs accessed October 28, 2025